Key Takeaways:
- The Golden Hour is the critical 60-minute window to deliver antibiotics when a child with cancer spikes a fever.
- Children with central lines (the tubes that deliver chemotherapy) are at high risk for serious infection because their immune systems are weakened.
- Cure 4 The Kids Foundation reduced its average time to antibiotics from 85 minutes to 33 minutes, exceeding national benchmarks.
- These results match or exceed outcomes from major academic children’s hospitals, achieved in an outpatient setting.
- The improvement came from frontline staff examining every step of the process and finding ways to move faster—no expensive technology required.
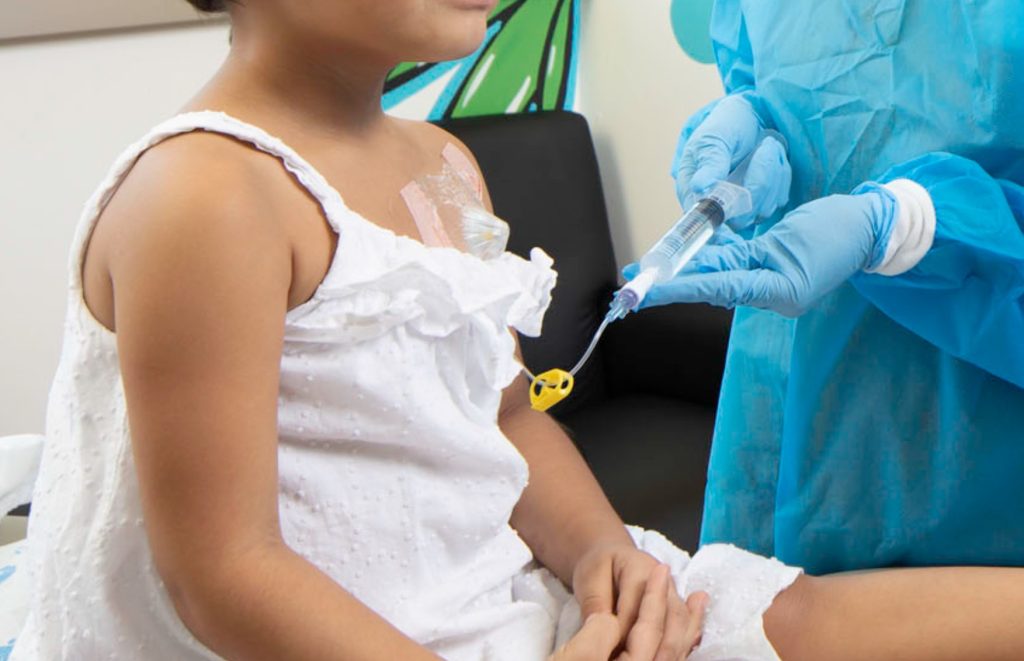
When your child spikes a fever during cancer treatment, time slows down and speeds up all at once. You’re watching the clock. You’re watching your child. You’re watching the team move around you and wondering if everything is happening fast enough.
I’ve talked to enough parents to know that feeling. It stays with them.
I want to tell you what our team has been doing to honor that urgency.
What Is the Golden Hour in Pediatric Oncology?
The Golden Hour refers to the critical 60-minute window to deliver antibiotics to a child with cancer who develops a fever. Because chemotherapy weakens the immune system, children with central lines—the tubes that deliver treatment directly into their bloodstream—are at high risk for serious infection. Their immune systems can’t fight infection the way ours can, and that’s why getting antibiotics started within 60 minutes can be lifesaving.
What is Febrile Neutropenia?
You may hear your care team use the term “febrile neutropenia.” This simply means fever in a child whose immune system has been weakened by chemotherapy. “Febrile” means fever, and “neutropenia” means the body has very few neutrophils, which are the white blood cells that fight infection. So, because your child’s body can’t fight infection on its own, febrile neutropenia is treated as a medical emergency.

Where We Started
I believe in being honest with our families, and with ourselves. So here’s where we were a year ago: our average time was 85 minutes.
To put that in perspective, nationally, roughly half of pediatric emergency departments consistently meet the 60-minute goal. Major academic children’s hospitals—institutions with 24-hour pharmacies, dedicated emergency departments, and teams of residents—report median times between 55 and 75 minutes. Some published studies show baselines over 90 minutes prior to launching improvement initiatives.
We weren’t behind. By national standards, we were in the middle of the pack.
But “middle of the pack” isn’t good enough when it’s your child with a fever. Our team knew we could do better.
Why Outpatient Specialty Centers Can Move Faster
Here’s something important to understand: hospitals and outpatient centers are built for different things.
Hospitals are designed to handle everything—trauma, surgery, complex inpatients, emergencies of every kind. That breadth is their strength. But it also means a child with a fever may be one of dozens of urgent situations competing for attention at any given moment.
An outpatient specialty center like Cure 4 The Kids Foundation is different. We do one thing: care for children with cancer and rare diseases. When a child with a central line spikes a fever, they’re not waiting behind a car accident or a cardiac event. Our team knows them. Their chart is already open. The pharmacist who will compound their antibiotic is thirty feet away, not in a central pharmacy serving an entire hospital.
Neither model is better. They serve different purposes. But for this specific situation—a known patient, a predictable emergency, a race against the clock—the focused outpatient model has real advantages. And our team has learned to use every one of them.

How Our Team Cut Time to Antibiotics in Half
This is the part that makes me proud.
A group of nurses, providers, pharmacists, and lab staff came together—not because anyone made them, but because they saw a problem and wanted to fix it. They walked through every step of the process. They asked hard questions. They challenged the way things had always been done.
They found minutes hiding everywhere. In the compounding suite. In the order sets. In the handoffs between teams. In the small hesitations that happen when people aren’t sure if they should ask for help.
And then they fixed them. One by one.
There was no magic solution. No expensive new technology. Just people who cared enough to look honestly at their work and commit to doing it better.
Where We Are Now: Cure 4 The Kids Foundation’s Golden Hour Results
Throughout 2025, we tracked every antibiotic administered to a patient with a central line.
Cure 4 The Kids Foundation’s Time to Antibiotics (2025)
We now consistently meet the Golden Hour benchmark. Some months, we’re well under it.
For context: These results put us on par with the best published outcomes from major academic children’s hospitals around the country—and we’re doing it as an outpatient clinic, without a 24-hour pharmacy, without a dedicated emergency department, without the infrastructure those institutions have.

Why I’m Sharing This
I’m not sharing this to brag. I’m sharing it because families deserve to know.
When I started Cure 4 The Kids Foundation in 2007, it was because I believed Nevada families shouldn’t have to leave home to get excellent care for their children. I’d seen too many families torn apart by distance—siblings separated, parents missing work, support systems left behind—because the assumption was that “real” pediatric cancer care happened somewhere else.
I wanted to build something that proved that wrong. This—what our team did in 2025—is that proof. Not because I told them to. Because it’s who they are.
To Our Families
If your child is being treated at C4K, here’s what I want you to know:
When your child spikes a fever, we feel the urgency too. We’re not just going through the motions. We’ve built systems to move fast, and we hold ourselves accountable to using them. We track our own performance—not because someone makes us, but because your child deserves that kind of attention.
You trusted us with the most precious thing in your life. This team takes that seriously. Every day. Every patient. Every minute.
To the Cure 4 The Kids Foundation Team
I’ve watched this organization grow from a kitchen table idea to what it is today. I’ve seen a lot of things I’m proud of.
This is near the top.
Not because of the numbers—though they’re remarkable. Because of what the numbers represent: a team that looked at itself honestly, identified where it could do better, and then actually did it. Without excuses. Without waiting to be told.
That’s the C4K Way. And you lived it.
When I started C4K, people told me Nevada couldn’t support this kind of care. That families would always have to leave.
This team just proved them wrong, 33 minutes at a time.
This is why I built C4K. This right here is the whole point.

About the Author: Annette Logan-Parker brings over 30 years of experience in pediatric oncology to her role as Founder and Chief Advocacy & Innovation Officer at Cure 4 The Kids Foundation. She has dedicated her career to improving outcomes for children with cancer and ensuring equitable access to cutting-edge treatments for all families.
























